In this issue, Issue 1/2024, Science
Eternal and ubiquitous – PFAS compounds in aquatic environments
Wastewater can provide us with a lot of valuable information about the health status of a community, the dynamics of infectious diseases or the consumption of specific groups of drugs, and even the consumption of psychoactive substances. Their analysis is also a source of information about the presence of persistent and potentially harmful substances such as per- and polyfluoroalkyl compounds (PFAS), known as perpetual pollutants.
PFAS and derivatives – characterization of compounds
Per- and polyfluoroalkyl substances are a class of more than 14,000. chemical compounds. Despite access to modern analytical techniques, the exact number of unique structures is still difficult to estimate. In October 2023. researchers have identified another 11 previously unknown compounds, which have been detected in the surface waters of the Great Lakes (USA), the North Sea and the Baltic Sea, among others [1].
PFASs have been in production since the 1950s. In the 1970s. and continue to be used in a wide variety of industrial and consumer products. Per- and polyfluoroalkyl substances are present in surfactants, foam and fire retardant coatings, as well as in textiles and food packaging. In addition, they have been detected in many products we use every day, including cosmetics and intimate hygiene products. Some of the chemicals will therefore end up in the sewage system. In March 2023. studies have been published that revealed that toilet paper is also a significant source of per- and polyfluoroalkyl substances [2]. They can appear in it from both wood-to-pulp processing and fiber recycling. The main compounds detected were polyfluoroalkyl di-phosphates (diPAP)-which can transform into more stable compounds such as perfluorooctanoic acid with potential carcinogenic properties [2].
What distinguishes these compounds is their chemical stability (due to the carbon-fluorine bond) and thermal stability, as well as their hydrophobic and lipophobic properties. By which many of them have toxic potential, bioaccumulative properties and high persistence and mobility in ecosystems [3]. Back in 2000. Concerns have been raised worldwide about the historically used long-chain perfluorinated alkyl acids (PFAAs), especially perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). Manufacturers in most developed countries have begun phasing out older compounds, including PFOA and PFOS, and producing fluorinated replacements with similar properties, such as short-chain PFAAs (perfluoroalkylcarboxylic acids) and per- and polyfluoroalkylether acids (PFEAs) [4]. In 2001. Giesy and Kannan published one of the first studies that highlighted the global distribution of perfluorooctane sulfonic acid (PFOS) due to the widespread use and production of PFAS compounds. PFOS has been identified in the tissues of wild animals – fish, birds and marine mammals, both in North America and Europe [5]. Around the same time, scientists also detected PFOS, PFOA, perfluorohexanesulfonate (PFHxS) and perfluorooctanesulfonylamide (PFOSA) in human blood serum [6].
Eternal pollution – sources and consequences of contact with it
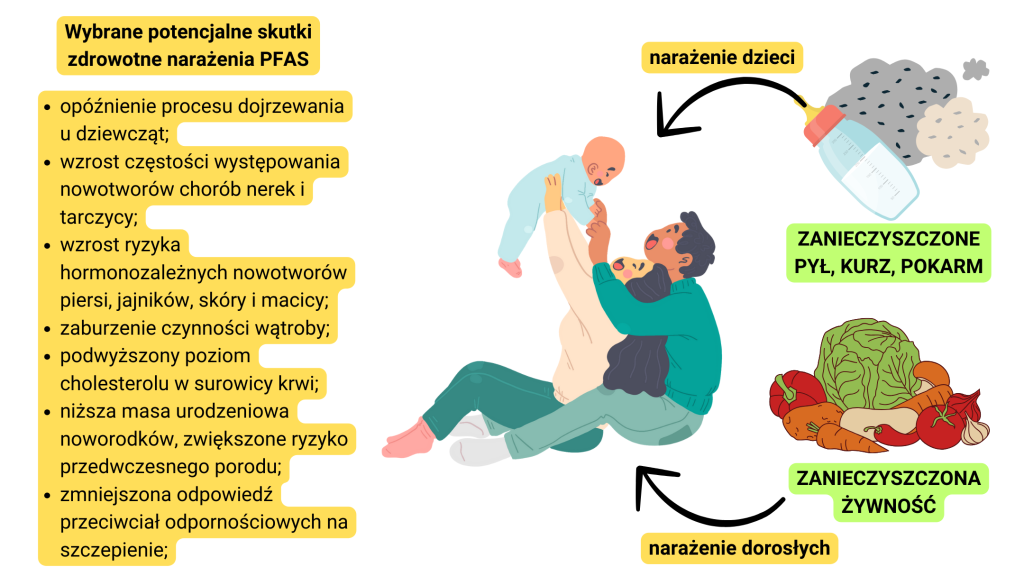
We are exposed to PFAS all the time, such as through direct contact with clothing [7]. However, the main route of adult contact with these compounds is through the consumption of food contaminated with PFOA and PFOS. An additional risk for children is contact with dust (see Figure 1). Contamination of the soil and water environment carries the risk not only of continued exposure to potentially harmful chemicals, but also of their bioaccumulation, as the serum half-lives of PFOS and PFOA are 5.4 and 3.8 years, respectively [9].
PFAS comes in two forms, long-chain and short-chain, which refers to the number of carbon atoms attached to the fluorine. The half-life of the compound in humans depends on the length of the chemical chain and is due to its ability to interact with various transporters involved in reabsorption processes in the liver, intestines and kidneys. Such physical and chemical properties as, among others. octanol-water partition coefficient PFAS, may affect their absorption capacity in humans [3]. Researchers have confirmed that short-chain PFASs are found primarily in wastewater, while long-chain PFASs are found mainly in sewage sludge, where they are more easily bound to solids [4].
In September 2020. European Food Safety Authority. The European Food Safety Authority has proposed a tolerable weekly intake standard for the sum of PFOA, PFOS, PFNA and PFHxS of 4.4 ng/kg body weight [12]. Today, we know that our exposure to these compounds in the aforementioned time frame may be higher, and established standards may not ensure health safety [3]. Currently, two PFAS are listed in the Stockholm Convention on Persistent Organic Pollutants (POPs). PFOS, including its salts and perfluorooctanesulfonyl fluoride (PFOSF), are listed in Appendix B (use restriction), and PFOA, its salts and related compounds (including precursors) are listed in Appendix A (elimination) [14]. In addition, the Commission for the Promotion of the Environment is also working on a new project. The POPs Review Committee recommended that PFHxS, its salts and related compounds also be included in the document. At the same time, the European Commission is considering banning some 10,000. substances from the group of persistent organic pollutants [15]. The European Union is taking this goal seriously. The production and use of perpetual pollution would be largely banned. The restrictions will be phased in from 2025. By the late 1930s. 21st century. Restrictions would also extend to imports into the Community of products containing PFAS. Since perpetual pollutants are used in almost all industries, the ban could also hit those areas we unequivocally associate as green, since per- and polyfluoroalkyl compounds are also present in solar panels or wind turbines. If the proposed ban were to go into effect, it would be the first ever case on this scale [14].
There is abundant epidemiological evidence that shows the harmful effects of PFAS on human health [8-11]. Their carcinogenicity, immunotoxicity, gene activation and developmental toxicity have been confirmed. Studies in communities exposed to elevated concentrations of compounds in this group have shown, among other things. endocrine disorders or increased risk of certain cancers, especially hormone-dependent cancers – breast, testicular, ovarian cancer (see Figure 1). Moreover, high exposure to per- and polyfluoroalkyl compounds has been linked to consumption of contaminated drinking water [3]. It is estimated to be the cause of as much as 75 percent near contamination sites. exposures. For this reason, we should pay special attention to the presence of these substances in the water environment – surface water, groundwater, tap water and wastewater.
PFAS in the aquatic environment – sources and concentrations
PFAS are released into the environment from two main sources: point and diffuse. The first are primarily industrial facilities, fire training sites (but also fires themselves), sewage treatment plants and landfills. Diffuse sources, often of unknown origin and location, are associated with a variety of phenomena – surface runoff, precipitation, decomposition of consumer products and atmospheric transport. Due to their high solubility in water and persistence of perpetual pollutants, oceans, groundwater and surface water are the main sinks for these compounds [16]. Studies have shown that European river systems are highly vulnerable. A review of studies from many areas of the world indicates that these compounds are present throughout the environment, regardless of the level of industrial or economic development. Which suggests that potential sources of PFAS in developing countries may be primarily household and consumer products, including personal care products [16]. Tests on the quality of surface water, used as a source of drinking water for millions of Germans, showed the highest concentrations in the Rhine, with a maximum of 2.8 mg/L. The content of the rest of the compounds in this group was considered background levels. In contrast, analyses in Sweden have found PFAS to occur in varying concentrations, potentially affecting the drinking water supplies consumed by 3.6 million residents [16].
The most glaring case of contamination with PFAS compounds occurred in the US. Drinking water in six water districts in two states has been contaminated with PFOA released by the nearby DuPont chemical plant near Parkersburg, West Virginia. The average concentration in the distribution system averaged 3.55 ng/ml (range 1.5-7.2 ng/ml), while in private intakes concentrations ranged from undetectable levels (<0.010 ng/ml) to as high as 14 ng/ml. In communities affected by contamination, there is still today a higher incidence of cancer and a higher percentage of children diagnosed with neuroatypism [16].
Bottled waters are also not free from perpetual contamination. The study found the presence of 10 PFAS compounds in 40 brands of spring and mineral waters sold in France. It has been estimated that the presence of compounds can affect 70 percent. of them. However, quantitatively, the researchers managed to determine six compounds in the range of 0.6-9.5 ng/l in 4 samples, with the total not exceeding 20 ng/l [16, 17].
PFAS in wastewater – what is the scale of the problem?
We know that PFAS flows into the treatment plant, but it also flows out in treated wastewater and sludge. Many studies indicate high levels of perennial pollutants in wastewater – above 100 ng/l [18]. PFAS emissions and incidence are expected to be higher in more urbanized and industrialized regions. However, studies show that in countries where production of these compounds is lower, their presence in the environment is offset by imports anyway. The complexity and diversity of the group of per- and polyfluoroalkyl compounds in the water and wastewater environment is primarily due to the fact that the wastewater is dominated by the so-called “per- and polyfluoroalkyl compounds”. precursors (from 33 to 63 percent of the sum of PFAS) – FOSA, FOSAA, FtOH and FTSA, which are able to transform into PFOA and PFOS. Precursors can change into two main compounds through biotransformation and oxidation. Biotransformation is driven by microorganisms and depends on the type of specific community that is present in biological reactors. Oxidation processes, on the other hand, require the use of advanced technologies. In both the first and second cases, we cannot confidently determine the degradation products or their level of toxicity. At least some of the precursors are transformed into compounds with higher harm to humans and the environment. In addition, recent studies suggest that shorter-chain PFASs behave similarly in terms of persistence and bioaccumulation to their longer-chain counterparts, which have been phased out of production. Also because some of them are the mentioned precursors. Short-chain perennial pollutants in wastewater are of particular concern because demand for them continues to increase dramatically, especially in developing countries, and it is likely that their environmental concentrations will be even higher in the future [18, 19].
Unfortunately, a characteristic feature of mechanical-biological treatment plants is the low removal efficiency of most PFAS pollutants [20]. Adjustment of various operating parameters (e.g., load, retention time, temperature, pH) is able to reduce the amount of these compounds in the wastewater, but then they accumulate in the sludge. Perennial contaminants have been confirmed in biosolids around the world, for example, analyses conducted in Australia have shown up to 910 ng/g. This continent is a particularly interesting case because most of the per- and polyfluoroalkyl compounds that enter the country are present in imported products; there is no major source of direct industrial emissions there [18]. PFASs have been shown to be absorbed by crops, so they can enter the food chain [21].
The future of PFAS
Not only is it necessary to continue analyzing the presence and quantity of PFAS in natural and anthropogenic ecosystems and to set strategic targets for reducing environmental emissions. But above all, further development of technologies to reduce PFAS migration into the water and wastewater environment. Since the currently researched technologies still do not extend beyond laboratories and pilot systems, they require significant investment support from both science and industry governing bodies.
Read also:“Perpetual Chemicals (PFAS) – investigated across Europe, look at the results.”
Dr.-Ing. Edyta Łaskawiec – water and wastewater technologist, assistant professor at the Department of Environmental Biotechnology, Silesian University of Technology
In the article, I used, among others. From the works:
[1] Kirkwood-Donelson K.I. et al, Uncovering per- and polyfluoroalkyl substances (PFAS) with nontargeted ion mobility spectrometry-mass spectrometry analyses, Science Advances, 9(43), 2023 https://www.science.org/doi/10.1126/sciadv.adj7048
[2] Thompson J.T. et al, Per- and Polyfluoroalkyl Substances in Toilet Paper and the Impact on Wastewater Systems, Environmental Science & Technology Letters, 2023 https://pubs.acs.org/doi/10.1021/acs.estlett.3c00094
[3] Dewapriya P. et al, Per- and polyfluoroalkyl substances (PFAS) in consumer products: Current knowledge and research gaps, Journal of Hazardous Materials Letters, 4, 2023 https://doi.org/10.1016/j.hazl.2023.100086
[4] Tavasoli E. et al, Distribution and fate of per- and polyfluoroalkyl substances (PFAS) in wastewater treatment facilities, Environmental Science: Processes & Impacts, 2021, https://pubs.rsc.org/en/content/articlelanding/2021/EM/D1EM00032B
[5] Giesy J.P., Kannan K., Global distribution of perfluoroacetate sulfonate in wildlife, Environmental Science & Technology, 2001, 35(7) https://pubmed.ncbi.nlm.nih.gov/11348064/
[6] Olsen G.W. et al, Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000-2015, Environmental Research, 2017, 157 https://doi.org/10.1016/j.envres.2017.05.013
[7] Ragnarsdóttir O. et al, Dermal uptake: An important pathway of human exposure to perfluoroalkyl substances?, Environmental Pollution, 2022, 307 https://doi.org/10.1016/j.envpol.2022.119478
[8] Domingo J.L., Nadal M., Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature, Environmental Research, 2019, 177 https://doi.org/10.1016/j.envres.2019.108648
[9] Rosen E.M. et al, Drinking Water-Associated PFAS and Fluoroethers and Lipid Outcomes in the GenX Exposure Study, Environmental Health Perspectives, 2022, 130(9) https://doi.org/10.1289/EHP11033
[10] Pinney S.M. et al, Exposure to Perfluoroalkyl Substances and Associations with Pubertal Onset and Serum Reproductive Hormones in a Longitudinal Study of Young Girls in Greater Cincinnati and the San Francisco Bay Area, Environmental Health Perspectives, 2023, 131(9) https://doi.org/10.1289/EHP11811
[11] Cathey A.L. et al, Exploratory profiles of phenols, parabens, and per- and poly-fluoroalkyl substances among NHANES study participants in association with previous cancer diagnoses. Journal of Exposure Science & Environmental Epidemiology, 2023, 33 https://doi.org/10.1038/s41370-023-00601-6
[12] PFAS in food: EFSA assesses risks and sets tolerable intake, dated September 17, 2020, https://www.efsa.europa.eu/en/news/pfas-food-efsa-assesses-risks-and-sets-tolerable-intake
[13] Stockholm Convention on Persistent Organic Pollutants, done in Stockholm on May 22, 2001. (Journal of Laws 2009 No. 14 item 76) https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20090140076
[14] The Reuters, No EU vote on restricting ‘forever chemicals’ before 2025, EU official says, from May 26, 2023 https://www.reuters.com/business/environment/no-eu-vote-restricting-forever-chemicals-before-2025-eu-official-says-2023-05-26/
[15] Kurwadkar S. et al, Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution, Science of The Total Environment, 2022, 809 https://doi.org/10.1016/j.scitotenv.2021.151003
[16] Crone B.C. et al, Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water, Critical Reviews in Environmental Science and Technology, 2019, 49(24) https://doi.org/10.1080/10643389.2019.1614848
[17] De Silva A.O. et al, PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding, Environmental Toxicology and Chemistry, 2021, 40(3) https://doi.org/10.1002/etc.4935
[18] Thompson K.A. et al, Poly- and Perfluoroalkyl Substances in Municipal Wastewater Treatment Plants in the United States: Seasonal Patterns and Meta-Analysis of Long-Term Trends and Average Concentrations, ACS EST Water, 2022, 2(5) https://doi.org/10.1021/acsestwater.1c00377
[19] Lenka S.P. et al, A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants, Water Research, 2021, 199 https://doi.org/10.1016/j.watres.2021.117187
[20] Hoang Nhat Phong Vo et al, Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation, Journal of Water Process Engineering, 2020, 36 https://doi.org/10.1016/j.jwpe.2020.101393
[21] Mroczko O. et al, Spatiotemporal patterns of PFAS in water and crop tissue at a beneficial wastewater reuse site in central Pennsylvania. Journal of Environmental Quality, 2022 https://acsess.onlinelibrary.wiley.com/doi/10.1002/jeq2.20408

 Polski
Polski







